This study has trial number NCT02078804 and is registered with ClinicalTrials.gov at https://clinicaltrials.gov/study/NCT02078804.
Eligibility criteria
We performed an individual RCT with a study base population from 18 out of 21 regions in Sweden17 comprising 74.5% of the total national population where CRC screening had not previously been offered (Stockholm, Gotland and Västernorrland regions were not included). Residents aged 60 years of age or who turned 60 in the year of randomization were identified from the Total Population Register maintained by the Swedish Tax Agency34. Individuals who had a previous diagnosis of CRC or anal cancer or who had participated in the NordICC trial were excluded8.
Consent
All individuals invited for screening signed a written informed consent for the procedure and for biobanking of samples. Individuals assigned as controls were not informed about study participation. The Stockholm Ethics Committee approved the study (2012/2058-31/3) and the review of medical charts (2015/1958-2). The Swedish Ethical Review Authority waived the need for informed consent for accessing pseudonymized register-based data (2022/01946-02 and 2022/06863-2).
Randomization and masking
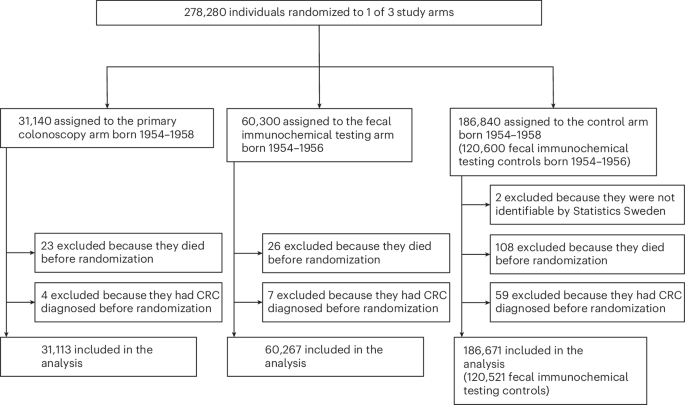
Between 11 February 2014 and 1 March 2016, a randomized block method was used to assign 201,000 individuals born 1954–1956 without prior CRC diagnosis to once-only primary colonoscopy, two rounds of FIT 2 years apart (FIT×2) or a usual care control arm with no organized program of screening activity (controls). All controls were intended for use in the separate comparisons of primary colonoscopy versus control and of FIT×2 versus control. Masking was not possible due to the nature of the trial. A list of all eligible individuals within each randomization block, defined by year of randomization, region of residence and sex, was obtained to randomly allocate individuals to the three arms. The target number of randomized individuals within the strata was determined based on the distribution of sex and county among 60-year-old individuals in Sweden (excluding the counties that did not participate in SCREESCO) in 2012. Because of low participation in the primary colonoscopy arm, an additional 77,280 individuals were randomized to primary colonoscopy or control between 30 May 2017 and 25 May 2018. We call the subgroup of controls randomized in 2014–2016 (120,600 controls) ‘FIT×2 controls’ because they are appropriate for use in the comparison against FIT×2 (also randomized in 2014–2016). All 186,840 controls were available for comparison against primary colonoscopy (randomized 2014–2018).
Interventions
All invitees in the primary colonoscopy and FIT×2 arms were sent a letter describing the study and a leaflet about CRC and screening. A reminder was sent after 8 weeks. No contact was made with individuals allocated to the control arm. In the primary colonoscopy arm, a second letter offered a scheduled colonoscopy or, if more convenient, a telephone appointment to schedule a colonoscopy. Individuals assigned to the FIT×2 arm were sent a set of kits for two stool samples each screening round. One central laboratory performed all FIT analyses using a single OC-Sensor DIANA automated analyzer (Eiken Chemical). A fecal hemoglobin concentration of ≥10 μg g−1 feces in either of the stool samples was deemed positive, triggering a colonoscopy invitation. All individuals in the FIT×2 arm, except those requiring colonoscopy surveillance after adenoma removal or after a CRC diagnosis, were offered a repeat FIT after 2 years, irrespective of participation in the first FIT screening round or the results of the first FIT. Colonoscopies were performed at 33 hospitals by 146 endoscopists, with a background training as gastroenterologists, surgeons or endoscopy nurses17.
Diagnostic phase of the trial
Individuals were randomized between 11 February 2014 and 25 May 2018 and subsequently invited to screening. Screening colonoscopies in the primary colonoscopy arm and the FIT×2 arm and FITs in the FIT×2 arm were performed between 2014 and 2020. We, therefore, consider the diagnostic phase of the trial to be between 2014 and 2020.
Usual care
In Sweden, all citizens have access to public healthcare35. A very small minority of individuals have private healthcare insurance on top of this (only 0.6% of Swedish healthcare is funded through insurance). During the study period, there was no national screening. Screening was performed in the Stockholm-Gotland healthcare region but not in the regions where SCREESCO was performed. In usual care, the main driver of colonoscopies is symptoms. During the study period, FIT has been introduced as an intermediate step in the investigation of symptoms to an increasing extent, where elevated hemoglobin in the fecal sample in a FIT taken because of symptoms triggers a colonoscopy. Individuals who are under surveillance due to increased CRC risk (that is, previous CRC diagnosis, inflammatory bowel disease or hereditary/familial CRC syndromes) may undergo colonoscopy during surveillance. Individuals may also be under surveillance after polypectomy of adenomatous colorectal polyps.
Primary and secondary outcomes of the trial
The ultimate primary endpoint of SCREESCO, for which the power and sample size calculations were performed, is CRC mortality (intervention versus control) at 15 years, and it will be reported later with follow-up until 31 December 2030. CRC incidence was listed as a primary endpoint in the study protocol, but this is formally a secondary outcome of the trial, along with an analysis of compliance, and exploratory outcomes include analyses of health economy, of colonoscopy quality and of the microbiome in feces.
Summary of changes to the SCREESCO study protocol and statistical analysis plan, 2013–2024
Changes in the study protocol version 2.0
The study protocol was amended after new power calculations due to an observed 35% participation in the colonoscopy arm (in Swedish, 10 March 2017; translated to English, 29 April 2021). The list of members of the Scientific Committee was updated.
Changes in the study protocol version 3.0
The study protocol was amended after the Scientific Committee decided on a last date of follow-up based on new power calculations. It was also decided that the previously described interim analysis would not be performed. The list of main publications was updated and so was the list of members of the Scientific Committee. A summary of changes to the study protocol and statistical analysis plan was added. Weblinks under ‘Head Secretariat’ were updated.
Changes in the statistical analysis plan 2.0
The statistical analysis plan was amended after new power calculations due to an observed 35% participation in the colonoscopy arm.
Changes in the statistical analysis plan 3.0
The statistical analysis plan was amended on 4 November 2024 after the Scientific Committee decided on a last date of follow-up based on new power calculations. It was also decided that the previously described interim analysis would not be performed. Details and clarifications regarding the initial and modified power calculations were added. The analysis of incidence of CRC was changed and is now based on cumulative incidence curves instead of the log-rank test.
Protocol deviations and rationale for the present study
Power calculations were performed for CRC mortality at 15 years alone. The initial study protocol and the current study protocol state, however, that the main research questions of SCREESCO are to investigate (1) if screening has an effect on the mortality from CRC, (2) if screening has an effect on the incidence of CRC and (3) what method should be used in Sweden regarding the effect according to (1) and (2). The Scientific Committee found a need for a timely assessment of baseline findings (diagnosed CRCs and adverse events during the diagnostic phase of the trial) that also includes the control arm (usual care) and diagnoses/events occurring in general (not only those directly related to SCREESCO screening colonoscopies). The present study is, therefore, listed as a planned main study (section 9, study protocol version 3). Note that CRC mortality is not assessed in the present study and that this main outcome of the trial will, instead, be presented in the final report of the trial with follow-up until 31 December 2030.
Note that this study does not constitute the interim analyses mentioned of CRC mortality at 5 years and 10 years of follow-up in the original study protocol and statistical analysis plan. An additional power calculation (statistical analysis plan version 3; Table 3) showed that there would be limited power to detect differences in CRC mortality at 10 years (follow-up until 31 December 2024) or earlier. Based on the calculation, the Scientific Committee decided that interim analyses of CRC mortality should not be performed.
The present study focuses on early CRCs and adverse events during the diagnostic phase and is listed as a planned analysis of the study. It builds upon a previous baseline assessment of screen-detected CRCs and adverse events in screening colonoscopies of SCREESCO17 and can be considered an extended baseline report or a 5-year interim analysis of diagnosed CRCs and adverse events occurring during the intervention phase. It includes extended information from Swedish health registers regarding CRCs diagnosed and adverse events in general in both screening arms and in the control arm during the diagnostic phase of SCREESCO when screening FITs and colonoscopies were performed.
Safety and adverse events
The endoscopy units reported serious adverse events that occurred within 30 days of the colonoscopy. A study nurse, together with A.F., checked the case report forms for completeness or inconsistencies and obtained additional information from the screening units if needed. Subsequent monitoring was, and will be, facilitated by follow-up using healthcare registers.
Measures of diagnostic yield and adverse events in the present analysis
In the present analysis of the diagnostic phase of SCREESCO, the outcomes were diagnostic yield of screening and usual care, in terms of CRC diagnoses (overall and by stage), and adverse events (cardiovascular and gastrointestinal). We also assessed death from any cause as a measure of overall health and a potential harm of screening.
Follow-up
In the present study, individuals were followed from randomization (2014–2018) until positive outcome in the corresponding analysis (CRC diagnosis or adverse event, respectively), emigration, death or end of the diagnostic phase (31 December 2020), whichever occurred first, through linkage between the Cancer Register, the Patient Register, the Total Population Register, the Cause of Death Register and the SCREESCO database.
Data on date of and stage at CRC diagnosis were extracted from the Swedish Cancer Register, to which registration is mandated by law29. Additional data on CRC stage were obtained from the Swedish Colorectal Cancer Register that, during 2008–2015, had a completeness of 98.5% for colon cancer and 98.8% for rectal cancer36. We defined CRC according to relevant International Classification of Disease (ICD) and Systematized Nomenclature of Medicine (SNOMED) codes registered in the Swedish Cancer Register. We used any of the following ICD 10th revision (ICD-10) codes for CRC: C18 (except C181), C19 and C20. We also required at least one of the following SNOMED version 3 (SNOMED/3; morphology code ICD-0/3) codes registered at the same time as the CRC: 81403 (adenocarcinoma), 82113 (tubular adenocarcinoma), 82133 (serrated adenocarcinoma), 82203 (adenocarcinoma in familial polyposis), 82433 (goblet cell adenocarcinoma), 82613 (adenocarcinoma arising from villous adenoma), 82633 (adenocarcinoma arising from tubulovillous adenoma), 84803 (mucinous adenocarcinoma) and 84903 (signet ring cell carcinoma/poorly cohesive carcinoma); or SNOMED/2 (SNOMEDO10; morphology code ICD-0/2): 81403 (tubular, villous or serrated adenocarcinoma), 82113 (tubular adenocarcinoma), 82203 (goblet cell adenocarcinoma or adenocarcinoma in familial polyposis), 84803 (goblet cell or mucinous adenocarcinoma) and 84903 (signet ring cell carcinoma/poorly cohesive carcinoma). We additionally included CRCs registered in the Swedish Colorectal Cancer Quality Register36.
The date of CRC diagnosis was defined as the first date of a CRC diagnosis in either of the two registries. Medical charts for individuals with CRC registered in SCREESCO, individuals who after a screening colonoscopy required further investigation or treatment (for example, computed tomography scan) and individuals whose polyp samples were sent to pathology were reviewed to assess the correctness of the CRC diagnosis and to extract additional information on stage. CRCs were considered screen detected if detected at colonoscopy (primary or secondary after a positive FIT) performed within SCREESCO.
Data on stage of CRCs detected at screening colonoscopy within SCREESCO were extracted from the SCREESCO database. Data on stage were also extracted separately for all individuals with a CRC diagnosis in the Swedish Colorectal Cancer Quality Register and/or the Swedish Cancer Register. We allowed for some administrative lag and included all entries in the Swedish Colorectal Cancer Quality Register and the Swedish Cancer Register occurring within 90 days from the first date of CRC diagnosis in either of the two registers. The TNM stages were obtained from the Swedish Colorectal Cancer Quality Register if the CRC was registered there and available (not missing) and, otherwise, from the Swedish Cancer Register if available. The Swedish Colorectal Cancer Quality Register contains both the clinical and pathological TNM, and the pathological TNM was used if available and, otherwise, the clinical TNM. Stage (I–II versus III–IV) was graded similarly to the American Joint Committee on Cancer system, which is based on the TNM classification, with stage I–II if T(any or missing)N0M0, T(0–4)N0M(0/missing) or T(0–1)N(0/missing)M0 and stage III–IV if N1, N2 or M1. Tx was considered as missing T stage and similarly for N stage and M stage. Stage was considered unknown if it did not fulfil any of the above.
Data on cardiovascular and gastrointestinal events (events related to colonoscopy—for example, bleedings and injuries) and on colonoscopies registered in an inpatient or outpatient setting were extracted from the Patient Register30. The Patient Register covers all inpatient care in Sweden, and the validity of this register has been found to be high, varying from 85% to 95% among different diseases30. The following ICD-10 codes were used to define gastrointestinal events: K922 (Unspecified gastrointestinal bleeding), S360 (Splenic injury), S365 (Colonic injury), S366 (Rectal injury), T810 (Bleeding iatrogenic) and T812 (Perforation). The following ICD-10 codes were used to define cardiovascular events: I20–I25 (Ischemic heart disease), I26 (Pulmonary embolism), I33 (Acute endocarditis), I46 (Cardiac arrest), I63 (Cerebral infarction), I74 (Peripheral artery embolism) and I81 and I82 (Venous thromboembolism).
Date of death was retrieved from the Cause of Death Register37.
Covariates
Biological sex (man/woman), year of birth, country of birth (Sweden versus other), region of residence, educational level, Charlson Comorbidity Index, a drug comorbidity index and history of any cardiovascular or gastrointestinal event and type of event (within 10 years prior to randomization) were used to describe individuals by arm. The Charlson Comorbidity Index was calculated based on the Patient Register in the last 10 years before inclusion, and the drug comorbidity index was calculated based on drug prescriptions registered up to 1 year before date of inclusion in the Prescribed Drug Register38,39,40. Country of birth was extracted from the Total Population Register, and educational level and region of residence were extracted from the Swedish Longitudinal Integrated Database for Health Insurance and Labor Market Studies (LISA).
Power calculations
The sample size was calculated based on the primary endpoint of CRC mortality. A priori, the study was powered to detect a 17.5% decrease in CRC mortality at 15 years in individuals invited to colonoscopy compared to the control arm and a 15% decrease in individuals invited to FIT compared to the control arm, based on an anticipated 1% cumulative CRC mortality between 60 years and 75 years of age. The original sample size target was 201,000 individuals.
Because of lower participation (35%) in the primary colonoscopy arm than expected (50%), new power calculations were performed to determine the additional number of randomized individuals needed to achieve acceptable power, and two additional age cohorts (born 1957 and 1958) were randomized to primary colonoscopy or control in a ratio of 1:6 (hence the deviation from the initially intended allocation ratio).
In the revised study protocol, we assumed a 15% disease-specific mortality reduction by 15 years of follow-up as a minimal clinically important effect in those invited to FIT×2 compared to the control arm, based on a participation rate of 50%, and a 17.5% disease-specific mortality reduction in those invited to colonoscopy compared to the control arm, based on a 35% participation. To allow these absolute risk reductions to be detected at a two-sided 2.5% significance level with 80% power for the comparison of FIT×2 versus control and 73% power for the comparison of primary colonoscopy versus control, the revised target sample size was 278,280 participants. The significance level was adjusted for two comparisons according to the Bonferroni method. In total, 31,140 individuals were randomized to the primary colonoscopy arm; 60,300 individuals were randomized to the FIT×2 arm; and there were two control groups: 186,840 controls to the primary colonoscopy arm, out of which 120,600 individuals also were controls to the FIT×2 arm (FIT×2 controls).
An additional power analysis was performed to determine when, in calendar time, the main analysis of SCREESCO would be performed because this was not clearly stated in the initial study protocol. The Scientific Committee decided that 31 December 2030 would be the last date of follow-up for the main analysis because power was not expected to meaningfully increase after this date.
Statistical analysis
In an intention-to-screen analysis, we report the number, proportion and incidence rate per 100,000 person-years of all screen-detected CRCs within SCREESCO and all other CRCs diagnosed in regular clinical practice during the diagnostic phase of SCREESCO, in total and by stage (I–II or III–IV). We similarly report incident cardiovascular and gastrointestinal events diagnosed in regular clinical practice and death from any cause. We compare each intervention arm with the corresponding control arm concerning each of the outcomes using incidence rates and IRRs using Poisson regression models with 95% CIs. Analyses were also performed separately in men and women, and a Poisson regression model with interaction between sex and study arm was used to compare the IRR in men versus the IRR in women under the null hypothesis of no difference (two-sided test of the interaction term). Note that only the FIT×2 controls (individuals randomized 2014–2016, during which individuals could be allocated to FIT×2) were used in the comparison of FIT×2 versus control in this study, whereas all controls (randomized 2014–2018, including the FIT×2 controls) were used in the comparison of primary colonoscopy versus control.
The full follow-up of up to a maximum of almost 7 years was used in the above analyses. In a complementary analysis, we also computed incidence rates and IRRs at each year of follow-up. Individuals were censored if and when they migrated out of Sweden. Individuals were similarly not considered at risk after their date of death. We assessed potential violations of equidispersion for each regression model and computed alternative CIs by use of robust standard errors. Because there were no signs of meaningful underdispersion or overdispersion and results were virtually identical, we did not report these analyses.
In a complementary analysis, we computed competing risk cumulative incidence curves of CRC (overall and by stage) where death from any cause was considered a competing risk.
We report baseline characteristics in participants and non-participants of the intervention arms along with diagnosed CRCs and cardiovascular and gastrointestinal events that occurred during the intervention phase. Individuals in the primary colonoscopy arm were considered to be participants if they underwent a screening colonoscopy, and individuals in the FIT×2 arm were considered participants if they returned a FIT in any of the two rounds.
Stata version 13.1 and R version 4.0.2 were used for power calculations. Analyses were performed using R version 4.0.2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.